"Synthesis and characterization of Na0.3RhO2 center dot 0.6H(2)O - a semiconductor with a weak ferromagnetic component",
- Authors
S. Park, K. Kang, W. Si, W.S. Yoon, Y. Lee, A.R. Moodenbaugh, L.H. Lewis, T. Vogt
- Journal
Solid State Communications
Vol.135, No.1, pp.51-56, 2005.07 - DOI
Abstract
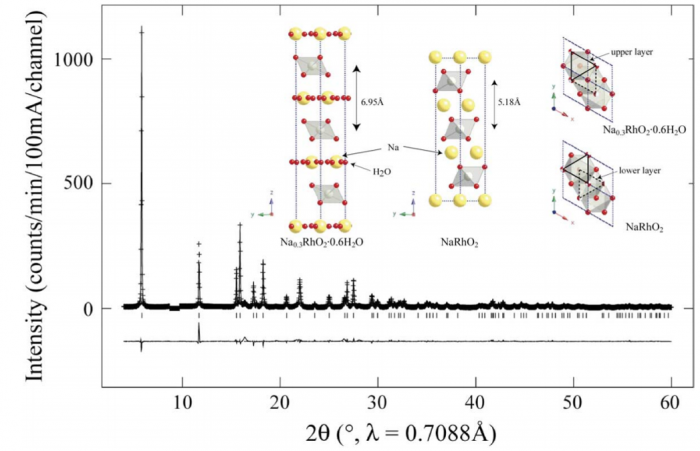
We have prepared the oxyhydrate Na0.3RhO2·0.6H2O by extracting Na+ cations from NaRhO2 and intercalating water molecules using an aqueous solution of Na2S2O8. Rietveld refinement, thermogravimetric analysis (TGA), and energy-dispersive X-ray analysis (EDX) reveal that a non-stoichiometric Na0.3(H2O)0.6 network separates layers of edge-sharing RhO6 octahedra containing Rh3+(4d6, S=0) and Rh4+ (4d5, S=1/2). The resistivities of NaRhO2 and Na0.3RhO2·0.6H2O (T<300) reveal insulating and semi-conducting behavior with activation gaps of 134 and 7.8 meV, respectively. Both Na0.3RhO2·0.6H2O and NaRhO2 show paramagnetism at room temperature, however, the sodium-deficient sample exhibits simultaneously a weak but experimentally reproducible ferromagnetic component. Both samples exhibit a temperature-independent Pauli paramagnetism, for NaRhO2 at T>50 K and for Na0.3RhO2·0.6H2O at T>25 K. The relative magnitudes of the temperature-independent magnetic susceptibilities, that of the oxide sample being half that of the oxyhydrate, is consistent with a higher density of thermally accessible electron states at the Fermi level in the hydrated sample. At low temperatures the magnetic moments rise sharply, providing evidence of localized and weakly-ordered electronic spins with effective moment per formula unit values of 2.0×10−1 μB for NaRhO2 and 0.8×10−1 μB for Na0.3RhO2·0.6H2O.